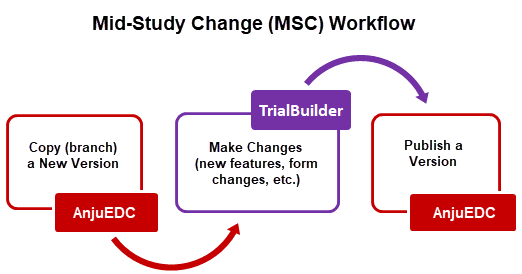
As shown in the workflow diagram, publishing a version is the last step after creating a new trial or performing MSCs. Once a trial is published, it is accessible via TrialMaster, where data collection can begin.
A trial is locked once it is published. A locked trial can no longer be edited, so it is important to have a duplicate unlocked trial to edit, if necessary.
After publishing, a trial should be tested to ensure proper functionality; changes can then be made and rolled out before migrating the study to production.
 Note:
AnjuEDC will NOT prevent you from publishing a trial where one or more
edit checks fail to compile in TrialBuilder (i.e., no error will occur
when the trial is published in AnjuEDC). However,
errors may occur in TrialMaster. Please ensure that any edit checks which
do not compile are either corrected or thoroughly tested.
Note:
AnjuEDC will NOT prevent you from publishing a trial where one or more
edit checks fail to compile in TrialBuilder (i.e., no error will occur
when the trial is published in AnjuEDC). However,
errors may occur in TrialMaster. Please ensure that any edit checks which
do not compile are either corrected or thoroughly tested.
To publish a version:
1. Access the Versions page. (See View Versions for guidance.)
2. Click the Versions link of the applicable trial under the Action column.
The Versions page displays.
3. Click the Publish link of the applicable version under the Action column.
The Publish dialog displays.
4. Do the following to complete the Publish dialog:
· Click the Finish button. The text box below the Target Version field displays the publishing status.
· When the text box displays "Publish Version Completed", click the Next button to migrate the version. The Migrate dialog displays.
 Note: To complete the migration
at a later time, click the X Close
button of the dialog and the Publish link remains enabled (in blue text).
When you are ready to migrate the version, simply click the Publish
link again and the Migrate dialog displays.
Note: To complete the migration
at a later time, click the X Close
button of the dialog and the Publish link remains enabled (in blue text).
When you are ready to migrate the version, simply click the Publish
link again and the Migrate dialog displays.
5. To migrate (merge) the previous version of the trial into the latest open version, do the following to complete the Migrate dialog. (The new trial version will merge new objects, deleted objects, and changes to objects between the two versions. Before migrating the versions, you can use the View Trial Differences Report to view the difference between the two versions.)
· The Change form states option determines if iCRFs will be reverted to Incomplete status after applying the migration, based on form structure changes made in the Mid-Study Change (MSC). This option is checked by default, meaning that forms will be reverted to Incomplete if the structure of the form has been updated in the MSC (e.g., by adding a new item or group). Note that forms retain their current status (and are not reverted to Incomplete) if this option is checked but the only change involved adding one or more hidden items (e.g., the Hidden property is set to true). Anju recommends unchecking the Change form states check box to prevent form status reversions to Incomplete strictly based on structural changes to the iCRFs. In this way, the system sets forms to Incomplete status based on validations firing on the forms after the migration, rather than based on form structure changes per se.
· Select the applicable Site check box(es). (To migrate all sites select the check box in the table header.)
· Click the Finish button.
· When the text box displays "Migrate Trial Data Completed", click the X button to close the dialog. The version you published is locked and the Publish link is disabled (black).
6. Click the Go Back button at the top left to return to the Trials page.
7. To start recording clinical data in TrialMaster, go to the Trials page and click the Conduct link of the applicable trial.